Navigating Compliance & Supply Chain Efficiency with Track & Trace Solutions
Here we look at specific ways trace and trace solutions help medical device companies tackle myriad supply chain and compliance challenges with ease.
Table of Content
For medical device companies, track and trace software plays a crucial role in ensuring regulatory compliance, enhancing supply chain visibility, and facilitating efficient recall management.
These platforms capture details about a product or asset, so you can track its status across the entire value chain.
It’s similar to the concept of establishing provenance for historical artifacts and artwork to verify authenticity and to trace records back to the original owner — be it an old master from the renaissance or a group of people robbed of their resources.
Here, that level of visibility enables medical device companies to navigate compliance challenges, safety recalls, and improve overall supply chain efficiency.
In this article, we’ll look at some of the more specific ways trace and trace solutions help medical device companies tackle myriad supply chain and compliance challenges with ease.
Track and trace software is a specialized solution designed to enable the end-to-end traceability of products as they move through the supply chain.
They typically use a range of technologies – RFID tags, serial numbers, barcodes, IoT sensors, and more – to capture and store information related to product identification, location, timestamps, and other relevant data points, creating a comprehensive audit trail.
Additionally, track and trace platforms typically handle tasks like associating products with serial or lot numbers, capturing events at different points in the supply chain, and gathering performance insights and analytics on individual assets.
Essentially, they provide an audit log of sorts that medical device companies can share with customers, regulators, and partners, use to improve asset performance, and quickly ID and fix issues that might put patients at risk.
One thing we should mention is, a lot of track and trace software was designed for one, specific purpose: helping medical device suppliers meet traceability requirements.
When track and trace platforms are siloed off from the rest of your business data, it becomes really difficult to enforce regulations, identify & isolate defective devices, or even ensure that supply chain ops are running efficiently.
Instead, track and trace capabilities must be embedded directly into your existing stack — starting with your ERP.
For example, in Dynamics 365, you can unify track and trace data with finance, operations, and CRM data. And, at the same time, still meet all the complex requirements that have forever defined the medical device space.
With all that in mind, let’s look at some of the specific ways fully-integrated traceability enables medical device orgs to tackle compliance and operating challenges with relative ease.
Medical device suppliers operate in a highly regulated environment, where adherence to strict regulatory requirements is critical.
Track & trace software helps suppliers maintain compliance by providing accurate and up-to-date documentation of product movement and traceability data.
Integrating traceability data into your finance and supply chain systems makes it easier to manage and securely share, and to ensure you’re always in compliance.
For example, if you’re using Dynamics 365, you can monitor and respond to regulatory inquiries, audits, and inspections from a single pane view. You can find and fix vulnerabilities that would otherwise undermine your standing with regulators. And, crucially, you can implement universal compliance policies across your entire network.
While D365 supports compliance by unifying your digital ecosystem so you can manage everything in one place, there are other Microsoft tools you can use to strengthen your compliance posture.
Microsoft Purview, for example, is a whole platform dedicated to safeguarding critical data – even in complex industries such as medical device manufacturing and distribution.
Purview’s Compliance Manager includes several tools that support compliance strategies. Inside, you’ll find over 360 pre-built regulatory templates you can use to create assessments based on actual regulations and quickly establish system-wide controls.
Below, you can see the platform’s Compliance Score feature, which generates a risk-based score based on the requirements defined in your regulatory templates. You’ll also be presented with suggested action items and solutions for improving your compliance situation.
You can also measure the impact of those prescribed measures, allowing you to prioritize improvements.
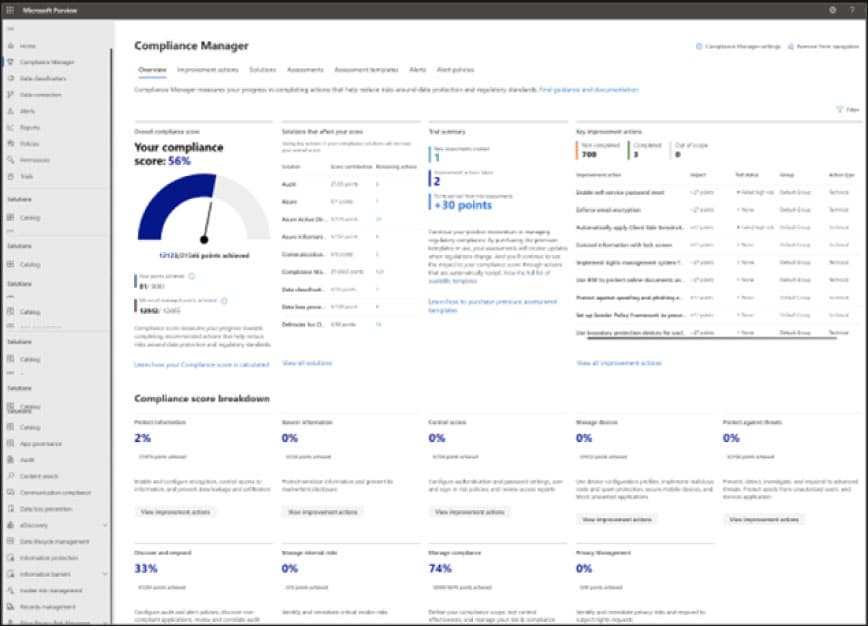
The platform also includes common control mapping, which lets you scale compliance efforts by applying controls to the entire system. And, a continuous regulatory updates feature, which automatically updates regulatory policies as new laws are codified and old requirements evolve.
Counterfeit medical devices pose significant risks to patient safety and brand reputation. Track and trace solutions prevent counterfeit devices from making their way to consumers by enabling product authentication at multiple checkpoints across the supply chain.
To do this, you’ll need to make sure you understand FDA guidelines for unique device identifiers (UDIs), which requires device manufacturers to provide identifiers in plain-text form and through automatic data capture technology such as RFID tags.
You’ll also need to make sure your strategy aligns with FDA rules for classifying devices, defining a manufacturer of record (the company developing the product), and establishing criteria for evaluating potential component suppliers.
D365 can help users meet these requirements, as well as prevent counterfeiting issues or tampering.
For example, Dynamics 365 Supply Chain Management includes a Product Information Management module that helps users identify products and product variants in the warehouse, on the production floor, and long after customers take them home.
You can create unique product numbers or IDs – either by entering numbers manually or using a number sequence generated automatically in the system.
When you first implement D365 SCM, you’ll need to come up with a good numbering system in order to prevent errors as well as improve traceability and logistics flows.
According to Microsoft, identifiers should ideally contain fewer than 10 characters, and no more than five classifying characters. You’ll also want to define dimensions for each product. This makes it easier to track individual devices and find products in the system using filters to narrow your search.
SCM users can also create barcodes, GTIN codes, and external product identifiers for devices that have been released to end-users.
If you’re using D365 Business Central, there’s an ISV app, COSMO UDI, designed specifically to help medical device manufacturers meet both FDA and EU requirements.
COSMO UDI includes data management, auditing, and labeling support. And – as you can see here, the app’s clean, straightforward interface makes it easy to define and edit UDI attributes at both the model and variant level.
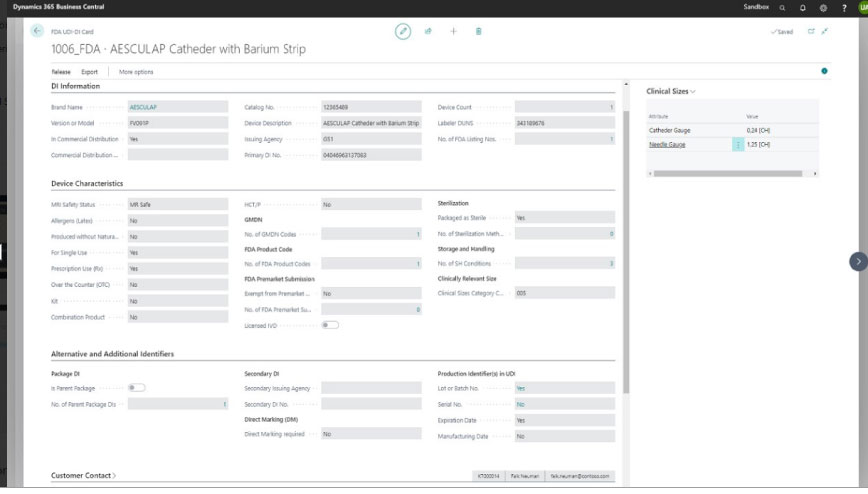
By verifying unique identifiers and analyzing them against authorized records, you can proactively detect and prevent the distribution of counterfeit devices. In turn, you can ensure patient safety and protect both your brand integrity and the bottom line.
Track and trace software provides real-time visibility into the location and status of medical devices as they move through the supply chain.
This visibility lets suppliers proactively monitor inventory levels and identify bottlenecks, deviations, and delays – allowing for real-time intervention.
Dynamics 365, of course, helps medical device orgs gain deep insight into operating processes, financials, device records, and other core business areas. When track and trace software becomes part of that unified stack, suppliers unlock new opportunities to improve.
One of our medical device clients, Irrimax, consolidated their fragmented legacy solutions into a unified ERP built on the Microsoft Cloud Platform and D365 Business Central, with integrated SCM and automation capabilities.
According to David Meyer, Irrimax VP of IT, the new system is set up so that it generates actionable insights for improving efficiency and productivity. They’re now working from accurate forecasts, and can run “what-if” scenarios that analyze things like how spikes in demand impact production requirements and supply chain operations.
On the D365 Finance & Operations side, JiwaMed offers an ISV solution that brings industry-specific functionality to medical device companies using the ERP.
Inside, you’ll find features for handling things like warranty claims, servicing, and inventory management, as well as pre-built processes that enforce industry best practices. Users can create role-based workspaces that provide easy access to critical tools and information, while also ensuring that accessibility doesn’t come at the expense of compliance.
Because JiwaMed is layered on top of D365 F&O, your ERP doubles as an end-to-end medical device management solution. And, with everything in one place, traceability data can be leveraged with the rest of your data to deliver actionable insights for improving supply chain performance.
Track and trace solutions also offer benefits beyond compliance and supply chain efficiency. They capture a wealth of data from across the entire supply chain — providing valuable insights businesses can use to optimize production processes and enhance the customer experience.
When combined with advanced analytics and reporting tools, medical device suppliers can monitor supply chain performance, identify trends, track KPIs, and make data-driven decisions to optimize operations.
Medical devices are an essential tool that support positive, long-term health outcomes. Meaning, if these devices have even the tiniest defects, the consequences can be devastating.
In the event of a product recall or safety alert, track and trace software helps medical device companies quickly ID and isolate the affected devices, by providing insights into their exact locations, as well as all parties involved in their distribution and use.
This enables suppliers to swiftly and accurately execute targeted recalls, minimizing the impact on patient safety, reducing costs, and protecting their brand reputation.
Trace and trace solutions support recall management with many of the same solutions used to mitigate the risk of counterfeit devices. So, UDIs, barcodes, data management, and so on. But, with recalls, you’ll also need to think about how to best communicate that information to patients, providers, and potentially, the general public.
Building on our last point about visibility, a unified platform brings track and trace data to everyone in your organization – including the sales, customer service, and marketing that communicate with customers directly.
That centralized approach allows companies to streamline their operations and avoid data silos, leading to better coordination and faster decision-making.
One solution, HCLTech IoT Platform for Healthcare Assets, is an ISV solution that extends the capabilities of D365 Project Operations. It covers a ton of ground, but basically, it’s a device ecosystem that comes with features like remote patient monitoring and lifecycle management, which could help suppliers mount a recall response with relative ease.
Bottom line, track and trace solutions allow medical device companies to control critical supply chain operations, mitigate risks, and enforce strict compliance requirements.
This, in turn, means suppliers can safeguard patient safety, get ahead of potential disruptions, and maintain a competitive edge in this challenging space.
Velosio is a veteran Microsoft Partner with a long history of working with medical device suppliers. Our experts can help you identify and implement the right D365 solutions. Plus, we’ll help you optimize that platform to ensure that it meets traceability and compliance requirements – and helps you reach more “traditional” business objectives like growth, agility, and scalability.
Contact us today to learn more about supply chain track & trace solutions.
Talk to us about how Velosio can help you realize business value faster with end-to-end solutions and cloud services.